Abstract
Background: Since the advent of Tyrosine kinase inhibitor (TKI) therapy, the survival of patients with chronic myeloid leukemia (CML) is approaching to that of general population. The European LeukemiaNet recommends monitoring reverse transcriptase quantitative PCR for the guidance of molecular response. However, the rare incidence of progression to blast phase may occur during frontline TKI therapy. The aim of this study is to identify prognostic factors for the progression to blast phase during frontline TKI therapy and to develop a nomogram to calculate the risk of transformation at 1 year.
Methods: We analyzed 347 patients with newly diagnosed CML in chronic phase (CP) and accelerated phase (AP) who were treated with frontline TKI therapy (standard-dose imatinib, 73 patients; dasatinib, 144 patients; and nilotinib, 130 patients) from July 2000 to September 2014. The presence of comorbidity was assessed by the Adult Comorbidity Evaluation-27. Given the rare incidence of transformation to blast phase and equivalent efficacy of second-generation TKIs between dasatinib and nilotinib, we compared the cumulative incidence of transformation to blast phase between imatinib 400 mg/day and second-generation TKI therapy. We performed univariate and multivariate competing risk regression to identify prognostic factors for cumulative incidence of transformation to blast phase with treatment failure (intolerance, resistance, and/or others) during frontline TKI therapy as a competing events. Multiple imputation was performed for missing variables to reduce bias. Nomogram was developed to estimate 1-year cumulative incidence of transformation to blast phase.
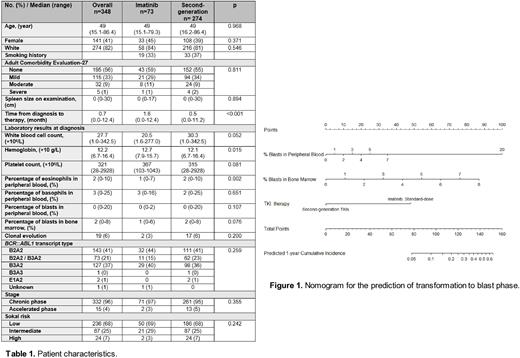
Results: The median age at the start of TKI therapy was 49 years (range, 15.1-86.4) with a median follow-up of 146 months (range, 5.3-253.9) (standard-dose imatinib, 231 months; dasatinib 137 months; nilotinib 138 months) (Table 1). Overall, the incidence of transformation to blast phase was observed in 8 patients during frontline TKI therapy; 4 patients received imatinib; and 4 patients received second-generation TKIs (1 patient, dasatinib; 3 patients, nilotinib). Competing risk regression identified percentage of blasts in peripheral blood and bone marrow, and standard-dose imatinib therapy as prognostic factors for transformation to blast phase during frontline TKI therapy. The 1-year cumulative incidence of transformation rates were 4% and 1% in the imatinib and second-generation TKI groups, respectively; the 5-year cumulative incidence rates were 5% and 1%, respectively (P=0.043 by Gray's test). The nomogram was developed for the risk calculations for 1-year transformation to blast phase (Figure 1).
Conclusion: The incidence of transformation to blast phase is rare in the era of TKI therapy. The nomogram helps physicians select optimal TKI therapy to prevent the transformation to blast phase. Given the dismal outcome of patients who progressed to blast phase, we propose frontline second-generation TKI therapy in patients with estimated 1-year cumulative incidence of transformation above 5% or higher.
Disclosures
Sasaki:Daiichi-Sankyo: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Otsuka Pharmaceuticals: Honoraria. Jabbour:Genentech: Other: Advisory Role, Research Funding; Amgen: Other: Advisory Role, Research Funding; Adaptive Biotechnologies: Other: Advisory Role, Research Funding; Spectrum: Research Funding; AbbVie: Other: Advisory Role, Research Funding; Pfizer: Other: Advisory Role, Research Funding; Takeda: Other: Advisory Role, Research Funding; Bristol Myers Squibb: Other: Advisory Role, Research Funding. Issa:Novartis, Kura Oncology, Nuprobe: Consultancy; Celgene, Kura Oncology, Syndax, Merck, Cullinan and Novartis: Research Funding. Garcia-Manero:AbbVie: Honoraria, Research Funding; BMS: Consultancy, Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Gilead Sciences: Research Funding; Astex: Consultancy, Honoraria, Research Funding; Aprea: Honoraria; Curis: Honoraria, Research Funding; Acceleron Pharma: Consultancy; Genentech: Honoraria, Research Funding. Kantarjian:NOVA Research: Honoraria; Novartis: Honoraria, Research Funding; KAHR Medical Ltd: Honoraria, Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Research Funding; Ipsen Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; ImmunoGen: Research Funding; Astellas Health: Honoraria, Membership on an entity's Board of Directors or advisory committees; Daiichi-Sankyo: Consultancy, Research Funding; Ascentage: Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding; Takeda: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal